Details of the Drug
General Information of Drug (ID: DMKBJWP)
| Drug Name |
Caffeine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CFF; Cafamil; Cafecon; Cafeina; Cafeine; Caffedrine; Caffein; Caffeina; Caffenium; Caffine; Cafipel; Coffein; Coffeine; Coffeinum; DHCplus; Dasin; Dexitac; Diurex; Durvitan; Enerjets; Ercatab; Guaranine; Hycomine; Kofein; Koffein; Mateina; Methyltheobromide; Methyltheobromine; Miudol; Nodaca; Organex; Percutafeine; Phensal; Stim; Teina; Thein; Theine; Tirend; Vivarin; Anacin Maximum Strength; Anhydrous caffeine; Caffedrine Caplets; Caffeina [Italian]; Caffeine Pure; Caffeine solution; Coffein [German]; Coffeinum N; Coffeinum Purrum; Component of Cafergot; DHC Plus; Dexitac Stay Alert Stimulant; Eldiatric C; GlaxoSmithKline Brand of Caffeine; Hycomine Compound; Keep Alert; Kofein [Czech]; Koffein [German]; Merck dura Brand of Caffeine; Methylxanthine theophylline; Midol Maximum Strength; Monomethyl derivative of Theophylline; Natural Caffeinum; Nix Nap; No Doz; Nodoz Maximum Strength Caplets; Passauer Brand of Caffeine; Percoffedrinol N; Pierre Fabre Brand of Caffeine; Quick Pep; Republic Drug Brand of Caffeine; Seid Brand of Caffeine; Theobromine Me; Theophylline Me; C 0750; Propoxyphene Compound 65; SK 65 Compound; TNP00310; Thompson Brand 1 of Caffeine; Thompson Brand 2 of Caffeine; Alert-pep; Anhydrous caffeine (JP15); Anhydrous caffeine (TN); Berlin-Chemie Brand of Caffeine; Bristol-Myers Squibb Brand of Caffeine; Cafcit (TN); Caffeine (USP); Caffeine (natural); Caffeine [BAN:JAN]; Caffeine, Monohydrate; Caffeine, anhydrous; Caffeine, synthetic; No-Doz; Pep-Back; Propoxyphene Compound-65; Quick-Pep; Refresh'n; SK-65 Compound; Tri-Aqua; Ultra Pep-Back; Wake-Up; CU-01000012617-3; P-A-C Analgesic Tablets; Theophylline, 7-methyl; Xanthine, 1,3,7-trimethyl; 1,3,7-Trimethyl-2,6-dioxopurine; 1,3,7-Trimethylpurine-2,6-dione; 1,3,7-Trimethylxanthine; 1-3-7-TRIMETHYLXANTHINE; 1-methyltheobromine; 3,7-dihydro-1,3,7-trimethyl-1H-purine; 7-Methyltheophylline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
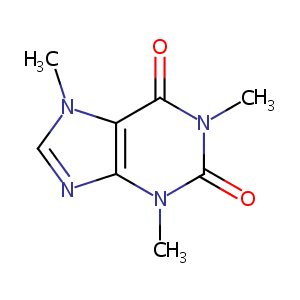 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 194.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Caffeine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Caffeine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 407). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006 Mar 8;295(10):1135-41. doi: 10.1001/jama.295.10.1135. | ||||
| 9 | Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004 Apr;61(7-8):857-72. | ||||
| 10 | Effect of cytochrome P450 (CYP) inducers on caffeine metabolism in the rat. Pharmacol Rep. 2007 May-Jun;59(3):296-305. | ||||
| 11 | CYP2E1 active site residues in substrate recognition sequence 5 identified by photoaffinity labeling and homology modeling. Arch Biochem Biophys. 2007 Mar 1;459(1):59-69. | ||||
| 12 | Determinants of interindividual variability and extent of CYP2D6 and CYP1A2 inhibition by paroxetine and fluvoxamine in vivo. J Clin Psychopharmacol. 1998 Jun;18(3):198-207. | ||||
| 13 | Monkey liver cytochrome P450 2C9 is involved in caffeine 7-N-demethylation to form theophylline. Xenobiotica. 2013 Dec;43(12):1037-42. | ||||
| 14 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 15 | Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab Dispos. 1997 May;25(5):617-22. | ||||
| 16 | PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012 May;22(5):389-95. | ||||
| 17 | A population and family study of N-acetyltransferase using caffeine urinary metabolites. Clin Pharmacol Ther. 1993 Aug;54(2):134-41. | ||||
| 18 | Product Information. Ocaliva (obeticholic acid). Intercept Pharmaceuticals, Inc., New York, NY. | ||||
| 19 | Carbo ML, Segura J, De la Torre R, et al "Effect of quinolones on caffeine disposition." Clin Pharmacol Ther 45 (1989): 234-40. [PMID: 2920498] | ||||
| 20 | Product Information. Naropin (ropivacaine). Astra USA, Westborough, MA. | ||||
| 21 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 22 | Brosen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S "Fluvoxamine is a potent inhibitor of cytochrome P4501A2." Biochem Pharmacol 45 (1993): 1211-4. [PMID: 8466541] | ||||
| 23 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 24 | Jonkman JH, Sollie FA, Sauter R, Steinijans VW "The influence of caffeine on the steady-state pharmacokinetics of theophylline." Clin Pharmacol Ther 49 (1991): 248-55. [PMID: 2007319] | ||||
| 25 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 26 | Broughton LJ, Rodgers HJ "Decreased systenuc clearance of caffeine due to cimetidine." Br J Clin Pharmacol 12 (1981): 155-9. [PMID: 7306430] | ||||
| 27 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 28 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 29 | Product Information. Rozerem (ramelteon). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 30 | Hartter S, Nordmark A, Rose DM, Bertilsson L, Tybring G, Laine K "Effects of caffeine intake on the pharmacokinetics of melatonin, a probe drug for CYP1A2 activity." Br J Clin Pharmacol 56 (2003): 679-682. [PMID: 14616429] | ||||
| 31 | Product Information. Lotronex (alosetron). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 32 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 33 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 35 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 36 | Product Information. Rilutek (riluzole). Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 37 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 38 | Bapiro TE, Sayi J, Hasler JA, et al. "Artemisinin and thiabendazole are potent inhibitors of cytochrome P450 1A2 (CYP1A2) activity in humans." Eur J Clin Pharmacol 61 (2005): 755-61. [PMID: 16261361] | ||||
| 39 | Product Information. Noroxin (norfloxacin). Merck & Co, Inc, West Point, PA. | ||||
| 40 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 41 | Krahenbuhl S, Sauter B, Kupferschmidt H, Krause M, Wyss PA, Meier PJ "Case report: reversible QT prolongation with torsades de pointes in a patient with pimozide intoxication." Am J Med Sci 309 (1995): 315-6. [PMID: 7771501] | ||||
| 42 | Beach CA, Mays DC, Guiler RC, et al "Inhibition of elimination of caffeine by disulfiram in normal subjects and recovering alcoholics." Clin Pharmacol Ther 39 (1986): 265-70. [PMID: 3948467] | ||||
| 43 | Granfors MT, Backman JT, Laitila J, Neuvonen PJ "Tizanidine is mainly metabolized by cytochrome P450 1A2 in vitro." Br J Clin Pharmacol 57 (2004): 349-53. [PMID: 14998432] | ||||
